

(The heat of sublimation of Na is 108 kJ/mol and ΔH of (NaCl) = −411 kJ/mol. By using Born Haber cycle for the formation of AgCl, calculate the lattice enthalpy of AgCl from the following data. THIS QUESTION IS BASED UPON BORN HABER CYCLE.Īn example is ice melting where the entropy will increase:The standard entropy, $S^$ is the enthalpy change when one mole of gaseous 2- ions are formed from one mole of its gaseous 1- ions. Other: Includes any assessment tools that do not logically fit into the above categories.Lattice enthalpy is a measure of the strength of these electrostatic forces.
#DO BORN HABER CYCLE PROBLEMS HOW TO#
Skill Demonstrations: All skill-based and physical demonstrations used for assessment purposes including skill performance exams.Įxams: All forms of formal testing, other than skill performance exams. Learning Objective: Learn how to predict relative lattice energies and to use the Born-Haber cycle.Topics: enthalpy, enthalpy of formation, lattice energy. Problem solving: Assessment tools, other than exams, that demonstrate competence in computational or non-computational problem solving skills. This is a degree applicable course but assessment tools based on writing are not included because this course includes essay exams that fulfil the writing component of the course.
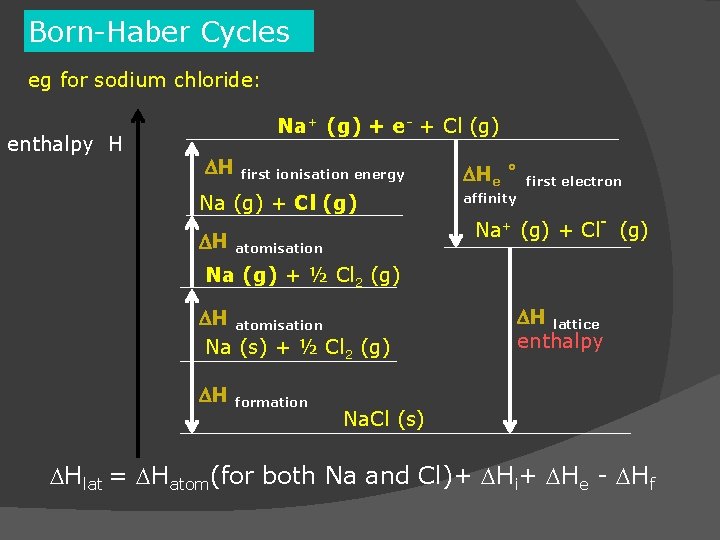
Writing: Assessment tools that demonstrate writing skill and/or require students to select, organize and explain ideas in writing. Student Learning Outcomes: Upon completion of the course, students will be able to: Untitled document It was also independently formulated by Kasimir Fajans and published concurrently in the same issue of the same journal. It was named after the two German scientists Max Born and Fritz Haber, who developed it in 1919. SRJC Equivalent Course(s): CHEM1A AND CHEM1B OR CHEM4A AND CHEM4B OR CHEM3A AND CHEM3AL AND CHEM3BĬertificate/Major Applicable: Both Certificate and Major Applicable The BornHaber cycle is an approach to analyze reaction energies. General Chemistry for Science Majors Sequence A SRJC Equivalent Course(s): CHEM1A OR CHEM4A OR CHEM3A AND CHEM3AL If you did not use the Madelung constant for fluorite for MgBr2, you would get. The importance of the zero-point energy contribution to the lattice energy in the case of the light metal. The result is U 0 2906.5 kJ mol 1 which may be compared with the Born-Haber cycle value of 2721.3 kJ mol 1.This difference indicates that there is an appriciable covalent contribution to the bonding. General Chemistry for Science Majors I, with Lab Use Born-Haber cycles calculations to show why formation of the salt MgBr. The lattice energy of MgH 2 has been calculated using a Born-Mayer model. Bond energy data are available only for the covalent bonds.

But, we cannot find bond energy data available for Na-Cl or Mg-O or Al-Cl.

We usually calculate Enthalpy of formation for substances using bond energy data. Repeatability: 00 - Two Repeats if Grade was D, F, NC, or NPĪRTICULATION, MAJOR, and CERTIFICATION INFORMATION Thermodynamics Born- Haber cycles Born Haber cycles Is when Hess’s Law is extended towards ionic compounds. Recommended: Course Completion of ENGL 1A or equivalent Prerequisites: Course Completion or Concurrent Enrollment in CHEM 3AL AND Course Completion of CHEM 42, or one year of high school chemistry taken within the last five years with a grade of B or higher AND Course Completion of MATH 154 or MATH 155 or MATH 156 or higher (MATH) or appropriate placement based on AB705 mandates


 0 kommentar(er)
0 kommentar(er)
